

 Environmental Toxicology
Newsletter
Environmental Toxicology
Newsletter
"Published Occasionally at Irregular Intervals"
~ Dr.
Arthur L. Craigmill ~
Extension
Toxicologist
Vol. 25 No. 1 -- March
2005
 "IN THIS ISSUE"
"IN THIS ISSUE"

Cow
Study Yields Surprises About Source, Amount of Dairy Air Pollution
California dairy cows produce only half the amount
of air pollutions had previously been believed and, perhaps more
important, most of a dairy cow's contribution to smog comes
not from
her manure, but from her belching, says the UC Davis
scientist
conducting the first controlled study of its kind.
Those unexpected findings may substantially
change the thinking and the practices of California regulators and
dairy operators trying to reduce air pollution.
"Our discovery means our whole approach to
dairy waste management and air-emissions management might change," said
Frank Mitloehner, the UC Davis air-quality specialist who is conducting
the study. "We have to re-think that the only good solutions are
engineering solutions, such as capping or aerating manure lagoons, and
consider biological avenues such as animal feeding and management."
"For the first time we can tell dairy farmers
the source of their air pollution," Mitloehner added. "For the most
tightly regulated pollutant, the 700 ozone-forming gases collectively
called volatile organic compounds, that source is not the cows' waste.
It's the cows."
For three months, Mitloehner has studied dairy
cows in controlled environmental chambers to collect precise
measurements of the volatile organic gas emissions they produce. The
information is urgently needed by the $4.6 billion, 1.5 million-cow
California dairy industry -- the largest in the world -- as dairy
producers try to comply with strict new pollution rules.
The dairy-air study is planned to last for two
more weeks, but the California Air Resources Board asked Mitloehner and
others to present their preliminary findings today at a meeting of the
San Joaquin Valley Air Pollution Control District in Fresno, CA.
The study was prompted by concern over air
quality in the San Joaquin Valley, which ranks as the worst in the
country. The No. 1 source of ozone (smog) air pollution in the valley
is exhaust emissions from trucks and cars. The No. 2 source is thought
to be gases from cows on dairy farms.
Using state-of-the-art air-collection and
analytical technology, and two environmental chambers to house the cows
in, Mitloehner precisely measured animal and waste production of
volatile organic gases and other pollutants like ammonia and methane.
He also videotaped the cows to correlate the timing of emissions with
their activities, such as eating, ruminating and excreting.
His preliminary findings indicate that cows and
their waste produce about 6.4 pounds of volatile organic compounds
(VOCs) per year.
The only previous estimate of total VOCs -- the
estimate that California's rigid new air standard is based on -- is
derived from a scientific study conducted in 1938. That old estimate
says that a cow produces 12.8 pounds of VOCs per year -- twice the
amount that Mitloehner found.
Furthermore, Mitloehner found that about 2.5
pounds of the total 6.4 pounds, or only about 40 percent, comes from
excreta.
REF: UC Davis News Service,


Potentially Harmful
Fluoride Levels Found in Some Instant Teas
Instant tea, one of the most popular
drinks in the United States, may be a source of
harmful levels of
fluoride, researchers at Washington University School of
Medicine in
St. Louis report. The researchers found that some regular strength
preparations contain as much as 6.5 parts per million (ppm) of
fluoride, well over the 4 ppm maximum allowed in drinking water by the
Environmental Protection Agency and 2.4 ppm permitted in bottled water
and beverages by the Food and Drug Administration.
The discovery stemmed from the diagnostic
investigation of a middle-aged woman suffering from spine pain
attributed to hyperdense bones. Testing for the cause of her symptoms
revealed the patient had high levels of fluoride in her urine. She then
disclosed a high consumption of iced tea -- claiming to drink one to two gallons of
double-strength instant tea throughout the day -- which led the
researchers to test for fluoride content in several brands of instant
tea available on grocery store shelves.
Each of the teas was tested as a regular-strength
preparation in fluoride-free water, and each contained fluoride, with
amounts ranging from 1.0 to 6.5 parts per million. The study is
reported in the January issue of The
American Journal of Medicine.
"The tea plant is known to accumulate fluoride from the soil and
water. Our study points to the need for further investigation of the
fluoride content of teas," says Michael Whyte, M.D.,
professor of medicine, pediatrics and genetics. "We don't know how much
variation there is from brand to brand and year to year."
In many communities in the United States,
fluoride is added to drinking water to help prevent tooth decay.
However, the Public Health Service indicates that the fluoride
concentration should not exceed 1.2 ppm.
Physicians have been aware that ingestion of high
levels of fluoride cause bone-forming cells to lay down extra skeletal
tissue, increasing bone density but also bone brittleness. The
resulting disease, called skeletal fluorosis, can manifest in bone
pain, calcification of ligaments, bone spurs, fused vertebrae and
difficulty in moving joints.
"When fluoride gets into your bones, it stays
there for years, and there is no established treatment for skeletal
fluorosis," Whyte says. "No one knows if you can fully recover from
it."
According to Whyte, the findings could aid in the
diagnosis and treatment of patients who have achiness in their bones.
In the future, doctors should ask such patients about their tea
consumption
REF: Washington University School
of Medicine, 25 January 2005, Whyte MP, Essmyer KE, Gannon FH, Reinus
WR. Skeletal fluorosis and instant tea. American Journal of Medicine
2005 Jan;118(1):78-82.


DPR Releases 2003
Pesticide Use Data;
Director Emphasizes Reduced-risk Strategy
The California Department of Pesticide
Regulation (DPR) reported a small
increase in commercial pesticide use
during
2003, compared to 2002. A DPR analysis linked the increase primarily to
wet, cool spring weather that caused more disease problems.
Some 175 million pounds of pesticide
applications were reported in
2003, a 4 percent increase from the previous year. Although analysts
said such variations are normal, DPR Director Mary-Ann Warmerdam will
launch an initiative to renew DPR emphasis on reducing pesticide risks
and use.
"Maintaining the status quo just isn't good
enough," said
Warmerdam, who joined DPR last September. "We must do more to reduce
risks and encourage IPM -- integrated pest management." (IPM promotes
least-toxic pest management with an emphasis on natural methods and
chemical use as a last resort.)
"DPR needs to expand its commitment to IPM with
a comprehensive,
long-term strategy that will maximize the use of our existing
resources, while seeking out new opportunities to support IPM on the
farm; in schools, parks and other public areas; and in our homes," said
Warmerdam.
Toward that goal, Warmerdam will direct DPR's
Pest Management
Advisory Committee (PMAC) to begin developing a blueprint for IPM
progress when the committee holds its next meeting on February 23.
Warmerdam noted that DPR budget cuts in recent years eliminated IPM
grants for agricultural and urban groups, and slowed advancement of
DPR's school IPM program.
"Meanwhile, the need for least-toxic pest
management methods has
never been greater," said Warmerdam. "Agriculture faces legal and
legislative mandates to improve air and water quality, while urban
areas are under similar pressure to reduce runoff and pesticide risks
in schools. IPM projects sponsored by DPR have already demonstrated
success in these areas," she said.
"Most importantly, our experience shows that
IPM is good for our
economy as well as our environment," said Warmerdam. "Pesticide users
who employ IPM save the time and expense associated with the use of
highly-toxic, highly-regulated pesticides. It's a win-win situation for
business, workers and the public, and for our air and water."
Warmerdam will ask PMAC to respond with an
initial set of
recommendations in six months. "We also will seek advice and support
from a broad range of stakeholders, including the environmental
community, industry, legislative representatives, and others," said
Warmerdam. "We all recognize the fiscal constraints facing government
and the private sector. But that is all the more reason to seek
environmental progress that can benefit our economy."
2003 pesticide use summary:
- Increases in pesticide pounds
applied from 2002 to 2003 were noted
in almonds (1.4 million pounds more, or 12 percent), strawberries (1
million pounds more, 12 percent), carrots (800,000 pounds more, 10
percent), rights-of-way (600,000 million pounds more, 16 percent), and
rice (500,000 pounds more, 9 percent).
- Decreased pounds applied were found
in wine grapes (600,000 pounds
less, or 3 percent), table and raisin grapes (600,000 pounds less, 3
percent), structural pest control (300,000 pounds less, 6 percent),
potatoes (300,000 pounds less, 12 percent), and lemons (200,000 pounds
less, 5 percent).
- Most-used pesticides as measured by
pounds were sulfur, petroleum
oils, metam-sodium, and methyl bromide. Sulfur use decreased slightly
but remained the most highly used pesticide, both in pounds applied and
acres treated. By pounds, sulfur accounted for 53 million pounds, or 30
percent of all pesticide use. It is a natural fungicide favored by both
conventional and organic farmers.
- Petroleum oil use decreased by
209,000 pounds; metam sodium use
decreased by 322,000 pounds, and methyl bromide use increased by
834,000 pounds.
- Use increased in most pesticide
categories. The largest increase
in pounds was with the fumigant 1,3-dichloropropene. (Fumigants are
applied at high rates, in part, because they treat a volume of space
rather than a surface area such as the leaves and stems of plants.)
- Chemicals classified as reproductive toxins increased in pounds
applied from 2002 to 2003 (up 480,000 pounds, or 2 percent) and
increased slightly in cumulative acres treated (up 22,000 acres, less
than 1 percent).
- A similar pattern applied to suspected carcinogens. Use of
these chemicals increased in overall pounds applied (up 1.9 million
pounds, 7 percent) and in cumulative acres treated (up 390,000 acres or
11 percent). The increase in pounds was mainly due to increase in uses
of the fumigant 1,3-dichloropropene but the increase in acres treated
was due mainly to use of the fungicides maneb, iprodione, mancozeb, and
captan.
- Use of organophosphate and carbamate chemicals, which includes
compounds of high regulatory concern, continued to decline by pounds,
decreasing by 680,000 pounds (about 8 percent). Treated acres were
nearly the same, down only 3,000 acres (0.05 percent). Use of
chlorpyrifos increased; the largest decreases in use were molinate,
thiobencarb, and diazinon.
- Use of chemicals categorized as ground water contaminants was
nearly the same in 2003 as in 2002. Use increased by 38,000 pounds
applied (less than 2 percent), but cumulative acres treated decreased
by about 5,000 acres (0.3 percent). Most of the increase in pounds was
in use of diuron and simazine.
- Chemicals categorized as toxic air contaminants, another
regulatory concern, increased by 2.6 million pounds applied (8
percent). Cumulative acres treated increased by about 367,000 acres (12
percent). Most of the increase in pounds was due to increased use of
methyl bromide and 1,3-dichloropropene. Most of the increase in acres
was due to maneb and 2,4-D.
- Use of reduced-risk pesticides increased considerably, by
311,000 pounds applied (41 percent) and 1.8 million acres treated (47
percent).
DPR analyses have shown that pesticide use varies
from year to
year depending upon pest problems, weather, acreage and types of crops
planted, economics, and other factors. The 2003 summary -- which
included analyses for 12 crops -- found pest problems for most were
higher in 2003 than in 2002, due to the wet and cool spring in 2003.
Prices for most of the 12 crops also improved in 2003. The threat of
higher financial losses may have prompted some growers to use more
pesticides.
Pesticide use is reported as the number of pounds of
active
ingredient and the total number of acres treated. Data for pounds
include both agricultural and nonagricultural applications; data for
acres treated are primarily agricultural applications. The number of
acres treated is cumulative; one acre treated three times is counted as
three acres.
REF: California Department of Pesticide Regulation News, January
26, 2005 (05-01)


DPR Reports on
Environmental Justice Project,
Releases Illness Statistics for 2003
The California Department of Pesticide
Regulation (DPR) announced a pilot project to monitor the air for
pesticides
in a
rural farm community. DPR also released a summary of statewide
pesticide injury data for 2003.
DPR Director Mary-Ann Warmerdam said the air
monitoring project in
Parlier, Fresno County, supports DPR's commitment to equitable
environmental protection for all California residents and workers.
"People who live and work closest to
agriculture deserve the same
high standard of environmental protection as other Californians," said
Warmerdam. "High environmental standards will help assure cleaner air
and water, better health for our children, and a sustainable future for
our agricultural economy."
Warmerdam said DPR will move forward
immediately with plans to
screen about two dozen pesticides during a 12-month period. First, a
local advisory group will be created to assist DPR staff with the
project. Monitoring is expected to begin this summer.
Parlier was chosen as the project community
from more than 80
potential sites around the state. The selection was selected based on
many factors, including the levels of pesticide use and the presence of
a significant ethnic population of children and adults. DPR's pilot
project is part of an environmental justice initiative launched by the
California Environmental Protection Agency.
DPR has a longstanding policy of focusing on
worker health and
safety, since those who work with or near pesticides face the greatest
exposure risk. Extending that focus to farm communities -- and ethnic
populations -- is a logical step, Warmerdam said.
While the Parlier study is not directly related
to DPR's pesticide
illness reporting program, Department scientists will study the Parlier
data to determine whether pesticide levels in the community's air could
pose excessive hazards. Ultimately, the data could also help DPR devise
steps to reduce pesticide exposures and illnesses.
Other DPR health and safety initiatives that
focus on rural and
ethnic populations:
- DPR is developing a community guide that will help rural
residents and others better understand their rights under the pesticide
regulatory system, including the illness complaint process.
- DPR redesigned and rewrote its Pesticide Safety Information
Series leaflets in 2004 to make them more easily understood by farm
workers. The 18 handouts in English and Spanish are available at County
Agricultural Commissioner offices or found online at <www.cdpr.ca.gov/docs/whs/psisenglish.htm>.
- In 2003, DPR helped the Fresno County Agricultural
Commissioner's office produce a series of worker safety videos in
English, Spanish, and Hmong.
- A 2004 survey by DPR and County Agricultural Commissioners
found that more than 10,000 California farm workers speak Punjabi, a
language of India. Worker safety leaflets will be translated into
Punjabi and distributed later this year.
- A training video for Mixtecs -- indigenous Indians from the
Mexican state of Oaxaca who have no written language -- was produced by
the Fresno Agricultural Commissioner with a $50,000 federal grant
secured by DPR. Tens of thousands of Mixtecs work in Central Valley
fields. The Mixtec videos were aired on a Fresno TV station in 2004
with a live, question-and-answer session. Copies of the video will be
made available for purchase this year.
2003 summary illness statistics
reported
DPR's newly-released summary of pesticide
illnesses showed a
decline from 2002 to 2003. Some 1,232 cases were investigated in 2003,
with pesticide exposure suspected or confirmed in 802 cases. In 2002,
there were 1,859 investigations, with 1,316 suspected or confirmed.
However, these statistics represent a range of illness incidents,
rather than a census of individual illnesses.
Of the 802 suspected or confirmed illnesses in
2003, some 405
(50.5 percent) involved the use of agricultural pesticides, and 397
(49.5 percent) involved non-agricultural pesticide exposure.
Occupational exposures accounted for 553 (69 percent) of the 802 cases.
DPR continues to emphasize the reporting of
pesticide drift
incidents, agricultural and non-agricultural. The number of suspected
or confirmed drift illnesses declined in 2003 compared to 2002 (256
cases and 33 episodes, compared to 478 cases and 39 episodes).
Non-occupational cases fell dramatically from
2002 to 2003 (725 to
303). That coincided with the end of a project in which California
Poison Control System (CPCS) phone operators provided DPR with illness
information from physicians. The project lapsed when a federal grant
ran out and DPR faced its own budget constraints.
Physician reporting is another factor in
non-occupational illness
statistics. For years, DPR researchers have highlighted problems with
physicians who fail to report suspected pesticide illnesses to their
county health officers within 24 hours, as required by state law.
Last fall, DPR began participating in a project
with the Office of
Environmental Health Hazard Assessment (OEHHA) to improve the
timeliness, quality, and completeness of illness reporting. Funded by a
$750,000 grant from the U.S. Environmental Protection Agency, the
project will seek to reestablish a working relationship with CPCS,
train physicians to better recognize and report suspected pesticide
illnesses, enhance reporting with Web-based tools, and create a
Web-based system for pesticide incident investigation in cooperation
with the County Agricultural Commissioners.
For a county-by-county breakdown of suspected
pesticide injuries
in 2003, see
REF: Department of Pesticide Regulation News,February 24, 2005
(05-04)


FDA Assesses New
Report on Acrylamide
The Food and Drug Administration (FDA) is reviewing a report
released on March 2, from the Food and Agriculture Organization and
World Health Organization Joint Expert Committee on Food Additives
(JECFA) on acrylamide in food. Acrylamide is a natural byproduct that
forms when certain carbohydrate-rich foods are fried, baked, or roasted
at high temperatures. Acrylamide can cause cancer in laboratory animals
at high doses, although it is not clear whether it causes cancer at the
much lower levels in food.
Since the discovery of acrylamide in food in
2002, FDA has initiated
a broad range of activities on acrylamide, including being at the
forefront of new toxicology research on acrylamide. This FDA research
includes the carcinogenicity and neurotoxicity studies and the
toxicology modeling work cited in the JECFA recommendations. The
results of these studies, expected in 2007, will be pivotal for future
evaluations of acrylamide.
Experts from FDA participated in the meeting and
recent FDA research
on acrylamide levels in food and acrylamide toxicology were used for
JECFA's evaluation. Although the report concludes that acrylamide may
be a human health concern, JECFA also cautions that there are
uncertainties in its conclusions because of limitations in the data
used to evaluate acrylamide. JECFA also made the following
recommendations that are consistent with the FDA's approach:
- Reevaluate acrylamide when ongoing carcinogenicity and long-term
neurotoxicity studies are available;
- Continue work on acrylamide using toxicology modeling;
- Continue appropriate efforts to reduce acrylamide concentrations
in food; and
- Encourage accumulation of scientific data on acrylamide in foods
in developing countries.
At this time, FDA advises consumers to eat a
balanced diet, choosing
a variety of foods that are low in trans fat and saturated fat, and
rich in high-fiber grains, fruits, and vegetables. FDA is also planning
to release new data this spring on acrylamide levels in the U.S. diet.
For further information about acrylamide, consumers can turn to the
FDA's Center for Food Safety and Applied Nutrition website at http://www.cfsan.fda.gov/~lrd/pestadd.html#acrylamide.
REF: FDA News Press Release, March 3, 2005

USDA Pesticide Data Program to
Release 2003 Data
The U.S. Department
of Agriculture's Agricultural Marketing Service announced that
the Pesticide Data Program Annual Summary, Calendar Year 2003 and
the 2003 data are available via the Internet at http://www.ams.usda.gov/science/pdp/download.htm.
Printed copies of the 2003 Annual Summary will be available in
mid-March. AMS is making the summary and the data available on the
internet in advance of publication.
Congress
approved implementation of the Pesticide Data Program (PDP) in January
1991 to improve the quality and quantity of information available on
chemical residues in domestically produced and imported food. Since the
program’s inception, PDP has a provided statistically-reliable test
data for 70 commodities including fresh and processed fruit and
vegetables, grains, fluid milk, butter, corn syrup products, pear juice
concentrate, meat and poultry, peanut butter and drinking water.
In 2003, the PDP
analyzed a total of 12,316 food and drinking water samples. The
commodities in the 2003 survey included 10 fresh fruit and vegetables
(asparagus, cantaloupe, cucumbers, mushrooms, onions, pears, spinach,
sweet bell peppers, sweet potatoes and tomatoes), six processed
commodities (canned asparagus, canned green beans, canned peaches, pear
juice concentrate/puree, frozen sweet corn, and frozen sweet peas),
barley, wheat flour and butter. Finished drinking water samples
were collected from community water systems in California, Colorado,
Kansas, New York and Texas. PDP also initiated a targeted survey
for the triazole class of fungicides and their metabolites in apples,
peaches (fresh and canned) and wheat flour.
The information below was taken from the Executive Summary:
Of the samples tested by multiresidue
methods, 43 percent of the fruit and vegetable samples, 8 percent of
barley samples, 45 percent of wheat flour samples, and 99 percent of
the butter samples had detectable residues. Residues detected in wheat
flour resulted primarily from low level detections of the triazole
alanine and triazole acetic acid metabolites. Residue findings in
butter were primarily low level residues of endosulfan sulfate and the
environmental contaminants dieldrin and DDE p,p’.
Overall, approximately 54 percent of all samples tested by multiresidue
methods contained no detectable pesticides (parent compound and
metabolite(s) is combined), 22 percent contained one pesticide, and 24
percent contained more than one pesticide. Generally, fewer pesticides
were found in processed products and grains than in fresh commodities.
Low levels of environmental contaminants were detected in cantaloupe,
cucumbers, spinach, and butter at concentrations below levels that
trigger regulatory actions.
In finished drinking water, PDP detected low levels (measured in parts
per trillion) of some pesticides, primarily widely used herbicides.
None of the detections exceeded established EPA Maximum Contaminant
Levels or Health Advisory levels.
PDP testing found residues exceeding an established tolerance in 0.3
percent of the 11,522 samples (excluding drinking water). A tolerance
is the maximum amount of a pesticide residue allowable on a raw
agricultural commodity. Established tolerances are listed in the Code
of Federal Regulations, Title 40, Part 180. Residues with no
established tolerance were found in 1.5 percent of all samples
(excluding drinking water). These residues were detected at very low
concentrations and may be the result of spray drift, crop rotations, or
the use of sanitizers in food handling establishments.
REF: USDA Agricultural Marketing Service, New Release, No.
037-05, March 1, 2005.

Lead Poisoning
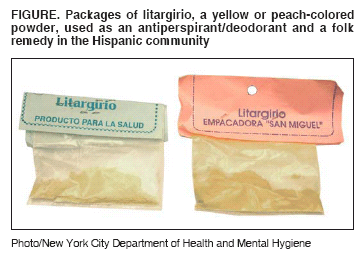
Associated with Use of Litargirio - Rhode Island, 2003
Lead can damage the neurologic, hematologic, and
renal systems. Deteriorated leaded paint in older housing remains the
most common source of lead exposure for children in the United States;
however, other lead sources increasingly are recognized, particularly
among certain racial/ethnic populations. In 2003, the Rhode Island
Department of Health (RIDOH) recognized litargirio (also known as
litharge or lead monoxide), a yellow or peach-colored powder used as an
antiperspirant/deodorant and a folk remedy in the Hispanic community,
as a potential source of lead exposure for Hispanic children. This
report summarizes a case investigation of elevated blood lead levels
(BLLs >10 µg/dL) associated with litargirio
use among two siblings in Rhode Island, the public health action taken,
and a survey of parents/guardians in three pediatric clinics in
Providence, Rhode Island, to assess litargirio use. Findings underscore
the importance of follow-up of elevated BLLs and thorough investigation
to identify all lead sources.
Case Report
In May 2003, RIDOH and the Health & Education Leadership for
Providence (HELP) Lead Safe Center investigated unexplained increases
in BLLs in twin Hispanic boys aged 7 years (twins A and B). Annual BLL
screenings for the twins since age 9 months were not elevated until
June 2001, when twins A and B had elevated BLLs of 14 µg/dL
and 15 µg/dL, respectively. Twin A's BLL increased to 42 µg/dL
in May 2003, despite completed remediation of interior lead paint
hazards in their home in June 2002 and of exterior lead hazards in May
2003, and provision of parental education about lead poisoning.
Similarly, twin B's BLL increased to 26 µg/dL during the
same period. In contrast, their younger brother's initial elevated BLL
of 17 µg/dL in August 2001, at age 9 months, decreased to
8 µg/dL by November 2002.
In May 2003, RIDOH and HELP Lead Safe Center staff conducted a home
inspection, which detected litargirio in a small glass jar in the
bedroom of the twins, who used the substance as an
antiperspirant/deodorant. The youngest brother did not use litargirio
and had a separate bedroom. After the litargirio tested positive for
lead by a sodium rhodizonate field test, all litargirio was removed
from the home, and a sample was sent to the state laboratory for
confirmatory lead testing. The litargirio sample contained 790,000
parts per million (ppm) (79%) lead. Follow-up BLLs decreased for twin A
(27 µg/dL in June, 22 µg/dL in August, and
13 µg/dL in November) and twin B (22 µg/dL
in June, 17 µg/dL in August, and 9 µg/dL in
November).
The twins' visiting grandmother from the Dominican Republic had
introduced litargirio into their home and also had given it to the
family of their two female cousins, aged 1 and 5 years. In June 2002,
the older girl had a BLL of 24 µg/dL, and the younger
girl had a BLL of 32 µg/dL. Previous annual BLL
screenings for the older girl were not elevated. In July 2002, after a
home inspection revealed lead paint hazards, their parents implemented
lead hazard control measures. However, the girls BLLs increased to 29 µg/dL
and 44 µg/dL, respectively, by January 2003. The older
sister used litargirio sporadically until the family ran out of the
product in January 2003, after which her BLLs decreased to 20 µg/dL
in March, 15 µg/dL in April, and 7 µg/dL in
November. Although the younger girl had not used litargirio, she shared
a bedroom with her older sister and likely ingested litargirio residue
on various surfaces through hand-to-mouth activity. Her BLLs also
decreased to 33 µg/dL in March, 29 µg/dL in
April, and 16 µg/dL in November after her sister
discontinued using litargirio.
Public Health Action
Litargirio is available locally in botanicas (i.e., shops selling
herbs) and bodegas (i.e., grocery stores) located in Hispanic
communities. It is manufactured and/or packaged by laboratories in the
Dominican Republic and sold in small, clear, plastic packets labeled
"litargirio" (See Figure Below). A litargirio sample purchased by RIDOH
staff from a local botanica contained 360,000 ppm (36%) lead.
Editorial Note:
Litargirio is used in the manufacture of batteries, glass, and
ceramics; in the vulcanizing of rubber; and as a paint pigment.
Dominicans, particularly those from rural areas, use it as an
antiperspirant/deodorant and as a traditional remedy for burns and
fungal infections of the feet. This report, the first to describe lead
poisoning associated with use of litargirio, demonstrates how a
thorough investigation of elevated BLLs led to the discovery of
litargirio, a previously unreported source of lead exposure.
Certain racial/ethnic populations at risk for lead exposure
through use of traditional or folk remedies might fail to disclose use
of these products when asked about use of "traditional or folk
remedies," rather than by product name. In this report, the twins'
mother repeatedly denied use of "traditional or folk remedies" because
she considered litargirio an ordinary product (i.e., deodorant), not a
remedy. RIDOH now inquires specifically about use of
litargirio when visiting Hispanic families of children with elevated
BLLs.
Data regarding dermal absorption of inorganic lead compounds in
humans is limited but reportedly substantially lower than absorption
through inhalation or ingestion. Although litargirio was applied to the
skin of these children, most of the product probably was ingested
through hand-to-mouth behavior after contact with the product or with
contaminated surfaces. Twin A, who had the higher BLL, sucked his
thumb, supporting this premise.
The survey results suggest that the prevalence of litargirio use in
Rhode Island was minimal. Later attempts by RIDOH staff to purchase
litargirio from botanicas or bodegas failed to locate any litargirio.
Because of these findings, RIDOH took no further action. Conversely, in
New York City (NYC), the NYC Department of Health and Mental Hygiene
was able to purchase litargirio from five of eight botanicas visited in
NYC after learning about the Rhode Island litargirio cases. One of the
five litargirio samples tested contained lead (430,000 ppm [43%] lead). A public warning was
issued, and botanica owners were required to remove all litargirio from
their stores.
REF: MMWR, March 11, 2005, 54(09).

Salmonellosis
Associated with Pet Turtles - Wisconsin and Wyoming, 2004
Salmonellosis associated with small pet turtles
in the United States was a major public health concern in the 1970s. In
1975, the Food and Drug Administration (FDA) banned commercial distribution of small
turtles (i.e., those with a carapace of <4 inches). The
FDA ban prevents an estimated 100,000 cases of salmonellosis among
children each year. However, a recent resurgence in the
sale of small turtles has generated concern. In Wisconsin and Wyoming,
at least six human cases of salmonellosis have been linked to such
turtles. This report describes the investigation into those
cases.
Wisconsin
Case 1. While vacationing with her family in Wisconsin in
late July 2004, a Kansas girl aged 4 years was taken to an emergency
department with diarrhea and fever of 4 days' duration. Her mother was
instructed to keep the child on a clear liquid diet until the diarrhea
ceased, and the child was released. The next day, the patient was taken
to an urgent-care clinic for treatment of bloody diarrhea, cramps, and
fever. Stool cultures yielded Salmonella enterica serotype
Pomona, a rare serotype. The child was placed on a 3-day course of
trimethoprim/sulfamethoxazole, and the illness resolved after 5 days.
Epidemiologic investigation by the Wisconsin Division of Public
Health (WDPH) determined that the
family had purchased a small turtle at a souvenir shop in northwest
Wisconsin. Warned by the public health nurse of the possible link
between the turtle and the child's illness, the family removed the
turtle, so the animal was not available for testing.
Cases 2 and 3. In July 2004, a boy aged 2 years was taken to
his physician with watery diarrhea and fever of 4 days' duration.
Twelve days later, his mother had onset of diarrhea and fever. The
physician counseled the patients; neither patient was treated and both
recovered completely.
Cultures of stool samples from both patients yielded S.
Pomona. Epidemiologic investigation by WDPH determined that the family
had recently purchased small turtles at a souvenir shop in
south-central Wisconsin. The family provided water specimens obtained
from the turtle habitat; these were cultured and yielded S.
Pomona.
Case 4. In August 2004, a boy aged 10 years was taken to an
urgent-care clinic with a 3-day history of diarrhea and vomiting. He
was hospitalized for 3 days and treated with antibiotics, after which a
stool specimen was obtained for culture; no pathogenic organisms could
be isolated. He subsequently had no symptoms for several months. In
November 2004, he was taken to an urgent-care facility after a 2-day
history of diarrhea and vomiting and was hospitalized for 3 days.
Stool specimens for culture yielded S. Pomona. Despite
negative cultures of stool specimens obtained 1 month after hospital
discharge, the child continued to have occasional loose, mucoid stools
as of January 2005.
An epidemiologic investigation by WDPH determined that the family
had purchased a small turtle from a souvenir shop during a vacation to
south-central Wisconsin in late July 2004; the mother could not recall
the name of the store. A week after the first hospitalization, the boy
heard media coverage about a link between a pet turtle and an ill
child. Consequently, the boy released
the turtle into a neighborhood creek. Thus, neither the turtle
nor its habitat were available for testing.
Public Health Response. In July 2004, WDPH began receiving reports
that small turtles were being sold or given away with purchase in
several tourist destinations in Wisconsin. WDPH sent a letter to all
local health departments on August 5 to alert them to this potential
health threat and asked local public health officials to stop the
distribution of turtles in their jurisdictions. Local health officers
were also asked to determine whether patients with salmonellosis had
any contact with reptiles, specifically turtles, and to provide
education for reptile owners. WDPH subsequently learned that at least
six souvenir shops in four Wisconsin counties were distributing
turtles. The public health alert and subsequent media coverage yielded
at least three cases (including case 4) of Salmonella infection
reported in young children who had recently purchased small turtles at
Wisconsin tourist destinations. The two most recent cases had onset
dates in February 2005 and are under investigation.
When analysis indicated that patterns from the patient and
turtle isolates associated with the first three Wisconsin cases were
indistinguishable, WDPH issued a press release on August 18,
2004, that identified the link between human cases of disease and
contact with pet turtles. The release also provided information on safe
handling of these animals and suggested options for surrendering the
turtles if owners chose not to keep them.
Once informed of the FDA ban by local health departments, most
Wisconsin retailers immediately discontinued selling small turtles. One
retailer refused to comply, stating that his turtles were free of Salmonella
and that he was distributing them for educational purposes only, which
was permissible under the FDA ban. The retailer produced a
report from a private laboratory indicating that cultures of cloacal
swabs obtained from 60 of a source batch of 10,000 turtles were
negative for Salmonella; the retailer claimed to be
distributing turtles that originated solely from this batch. Local
health officials informed the retailer that, because of the
intermittent nature of bacterial shedding, the results did not ensure
that all of the turtles were free of Salmonella and that their
distribution was illegal, regardless of their carrier status. The
retailer refused to comply with the order from the local health
department and continued to distribute the animals. WDPH issued an
emergency order on August 19 directing him to terminate any public
distribution of small turtles.
The retailer contacted a laboratory that agreed to test the turtles
and submitted samples from six of his turtles. Cloacal swabs from one
turtle yielded a mixture of S. Pomona and S. enterica
serotype I. The retailer stopped distributing turtles on August 24 and
returned the remaining animals to the supplier.
When specimens from the patient in case 4 were tested in November
2004, the banding pattern of the PFGE supported an epidemiologic link
among all four patients. Although slight differences existed in the
banding pattern between this last patient and the cloacal sample from
the turtle, epidemiologic and laboratory evidence supported the
conclusion that the illnesses in all four cases were the result of contact with turtles.
Wyoming
Case 1. In July 2004, a woman aged 80 years from central
Wyoming visited her health-care provider with a 5-day history
of fever, severe diarrhea, and increased urinary frequency. Cultures of
urine, feces, and blood all yielded S. enterica serotype Typhimurium.
The patient was hospitalized for 5 days, then discharged to a
transitional care unit for an additional 9 days. She received
intravenous (IV) antibiotics for 10 days during her stay in the
hospital and transitional care unit. At the time of discharge, her
condition had improved.
Investigation by the Casper-Natrona County Health Department (CNCHD)
determined that the woman lived with her daughter and the extended
family owned a turtle, but the woman had no known direct contact with
the turtle. However, the turtle bowl was cleaned in the family kitchen
sink. Cultures of environmental samples obtained from the turtle
habitat grew S. Typhimurium. PFGE patterns of environmental
and patient isolates tested at the Wyoming Public Health Laboratory
were indistinguishable.
Case 2. In August 2004, a boy aged 6 years from west-central
Wyoming visited his health-care provider with a 3-day history of
nausea, diarrhea, and vomiting. Blood cultures were negative, but a
stool sample yielded S. Typhimurium.
Wyoming Department of Health staff visited the boy's home 7 days
after illness onset. His mother reported that the family owned two pet
turtles. The boy was allowed to handle the turtles, but his mother fed
them and cleaned their aquarium because she was aware of the
risk for Salmonella infection.
Specimens for culture were obtained from the turtles and their
living environment. All samples yielded S. Typhimurium and
were indistinguishable from the patient's sample by PFGE. The samples
did not match the patterns of those from case 1.
Both turtles had been purchased from the same pet store, which had
been contacted by CNCHD on two previous occasions regarding its illegal
sale of turtles. The pet store informed CNCHD that the turtles were
being used solely for educational purposes. After investigating the two
cases of human salmonellosis, CNCHD confiscated the remaining turtles
from store C. CNCHD publicized this event to discourage future sales of
small turtles and to inform the public about the risk for
salmonellosis. The Wyoming Department of Health plans to mail an
informational packet about reptiles and Salmonella to all pet
stores in the state in summer 2005.
Editorial Note:
Salmonella infections usually result in a mild,
self-limiting gastroenteritis but can also lead to severe invasive
illness, such as septicemia or meningitis, especially in infants and
immunocompromised persons. Reptiles are a well-recognized source of
human salmonellosis, maintaining fecal carriage rates of Salmonella
of >90%. Contact with reptiles and amphibians accounts for an
estimated 74,000 (6%) of the approximately 1.2 million sporadic human Salmonella
infections that occur annually in the United States.
Successful management of turtle-associated salmonellosis requires
public health investigations to incorporate laboratory, epidemiologic,
environmental health, and policymaking components. When investigating
cases of salmonellosis, health officials should consider patient
contact with reptiles and take action to ensure that vendors and stores
do not distribute small turtles illegally. Additional information about
safe ownership of reptiles is available at http://www.cdc.gov/healthypets/animals/reptiles.
REF: MMWR, March 11, 2005, 54(09).

 TOXICOLOGY
TIDBITS
TOXICOLOGY
TIDBITS 
Herbal
Tea for Infant Colic Unsafe
A soothing cup of herbal tea with star anise has
traditionally been
heralded as an easy way to calm a colicky baby. But before your child
takes a sip, consider this. Contaminants
in the herbal tea can cause
dangerous neurological problems in infants.
In recent years, mounting evidence has indicated
cross-contamination between Chinese star anise (Illicium verum), which is
considered generally safe for consumption, and the closely related
species Japanese star anise (Illicium
anisatum). The Japanese version of star anise contains potent
nerve toxins. (Ed. Note: One of these toxins is anisatin, which is
a GABA neurotransmitter antagonist.) Physicians reporting in the
journal Pediatrics treated seven babies, aged 2 to 12 months, with
signs of star anise poisoning over a two-year study period. Symptoms of
toxicity in these infants included jitteriness, vomiting, irritability,
jerky movements, and seizures. All infants had received at-home herbal
tea with star anise at least once, although the doses may have varied
in all cases from one star to six anise stars boiled in water, and
given to the infants as little as once per day to as much as four times
per day. Analysis of samples of the anise star herbs used to make the
herbal tea showed toxic compounds, some at very high levels. All of the
infants experienced complete recovery within 48 hours of treatment.
Barbara M. Garcia Pena, MD, MPH, and co-authors say
the toxicities
found in the infants could be due to an overdose of Chinese star anise
(which at high doses can be toxic to the nervous system), contamination
with the Japanese star anise, or a combination of the two.
"Star anise tea should no longer be given to infants
because of its
potential danger in this population," the authors conclude in the
journal report. On Sept. 10, 2003, the U.S. Food and Drug
Administration warned
consumers not to buy herbal teas brewed from star anise. The statement
read: "It has come to FDA's attention that brewed "teas" containing
star anise have been associated with illnesses affecting about 40
individuals, including approximately 15 infants."
Star anise tea is also marketed under the name Anise
Estella.
REF: November 12, 2004, FSnet Nov. 15/04


Acute Illness from Dry
Ice Exposure During Hurricane Ivan
- Alabama, 2004
Natural disasters such as hurricanes often impair
delivery of
essential services, including electricity. When normal refrigeration
methods are unavailable, affected populations seek alternative means of
protecting perishable foodstuffs. One alternative is to use frozen
carbon dioxide (CO2) (i.e., dry ice).
In September 2004, in anticipation of a power
outage during the aftermath of Hurricane Ivan, a man aged 34 years in
Mobile, Alabama, purchased a 100-lb block of dry ice from a local ice
house. The block of dry ice was divided into four equal parts and
packaged in brown paper bags, which were placed in the front seat of
the man's pickup truck. The windows were closed, and the air
conditioner was set to recirculate air inside the cab of the truck.
After driving approximately one quarter mile from the ice house, the
man had shortness of breath; his breathing difficulty increased as he
drove the next mile. The man telephoned his wife and asked her to call
911. He then pulled his truck into a parking lot, parked, and lost
consciousness. His wife drove to the parking lot and located her
husband's truck; immediately after she opened the door to the vehicle,
her husband began to awaken. Emergency medical services
personnel arrived soon afterward. They determined that the man's vital
signs were normal and he required no further medical evaluation.
Although the man complained of a headache for the next 24 hours, he
recovered completely.
Dry ice has a temperature of -109.3ºF
(-78.5ºC) and can be used to keep perishable foods
cold. As dry ice melts, it undergoes sublimation (i.e., direct
conversion from a solid into gaseous CO2, bypassing the
liquid state). Improper ventilation during use, transport, or storage
of dry ice can lead to inhalation of large concentrations of CO2
with subsequent harmful effects, including death. Previous reports have
described illness and death caused by occupational exposures and
unintentional nonoccupational exposures to dry ice in enclosed spaces
such as automobiles and submarines. Because CO2 is colorless
and odorless, persons who transport, use, and store dry ice must be
educated about its potential dangers.
In the case described in this report, the man did
not receive any
warnings from the ice house regarding the potential danger of CO2
exposure from dry ice. If the air conditioner had not been set to
recirculate air inside the cab of the truck, the CO2 poisoning
symptoms might not have occurred. In addition, placing the ice in the
bed of the man's truck would have reduced exposure.
REF: MMWR, December 24, 2004 /
53(50);1182-1183


Fatal Rat-Bite Fever - Florida and
Washington, 2003
Rat-bite fever (RBF) is a rare, systemic illness
caused by infection with Streptobacillus moniliformis. RBF has
a case-fatality rate of 7%-10% among untreated patients. S.
moniliformis is commonly found in the nasal and oropharyngeal flora
of rats. Human infection can result from a bite or scratch from an
infected or colonized rat, handling of an infected rat, or ingestion of
food or water contaminated with infected rat excreta. An abrupt onset
of fever, myalgias, arthralgias, vomiting, and headache typically
occurs within 2--10 days of exposure and is usually followed by a
maculopapular rash on the extremities. This report summarizes the
clinical course and exposure history of two rapidly fatal cases of RBF
identified by the CDC Unexplained Deaths and Critical Illnesses (UNEX)
Project in 2003. These cases underscore the importance of 1) including
RBF in the differential diagnoses of acutely ill patients with reported
rat exposures and 2) preventing zoonotic infections among persons with
occupational or recreational exposure to rats.
REF: MMWR, January 7, 2005, 53(51 &
52);1198-1202.

Surveillance
Summaries: Cryptosporidiosis
and Giardiasis
Cryptosporidiosis Surveillance -
United States, 1999-2002
Cryptosporidiosis is a gastrointestinal illness
caused by
protozoa of the genus Cryptosporidium. During 1999-2002, the
total number of reported cases of cryptosporidiosis reported to CDC
increased from 2,769 for 1999 to
3,787 for 2001 and then decreased to 3,016 for 2002. The number of
states reporting cryptosporidiosis cases increased from 46 to 50, and
the number of states reporting more than four cases per 100,000
population increased from two to five. A greater number of case reports
were received for children aged 1-9 years and for adults aged 30-39
years compared with other age groups.
Incidence of cryptosporidiosis was particularly high in the upper
Midwest and Vermont. Peak onset of illness occurred annually during
early
summer through early fall.
Transmission of cryptosporidiosis occurs throughout
the United States,
with increased diagnosis or reporting occurring in northern states.
However, state incidence
figures should be compared with caution because individual state
surveillance systems have varying
capabilities to detect cases. The seasonal peak in age-specific case
reports coincides with the summer recreational water season and might
reflect increased use of communal swimming venues (e.g., lakes, rivers,
swimming pools, and water parks) by young children.
Giardiasis Surveillance - United
States, 1998-2002
Giardiasis is a gastrointestinal illness caused by
the protozoan parasite Giardia intestinalis. During 1998-2002,
the total number cases of giardiasis reported to CDC decreased from
24,226 for 1998 to 19,708 for 2001 and then increased to 21,300 for
2002. The number of states reporting
giardiasis cases increased from 42 to 46; however, the number of states
reporting more than 15
cases per 100,000 population decreased from 10 to five. A greater
number of case reports were received for children aged 1-9 years and
for adults aged 30-39 years compared with other age groups. Incidence
of giardiasis was highest in northern states. Peak onset of illness
occurred annually during early summer through early fall. The increase
observed for 2002 might reflect increased reporting after reporting of
giardiasis as a nationally notifiable disease began
in 2002. Transmission of giardiasis occurs throughout the United
States, with increased diagnosis or reporting occurring in northern
states. However, state incidence figures should be compared with
caution because individual state surveillance systems
have varying capabilities to detect cases. The seasonal peak in
age-specific case reports
coincides with the summer recreational water season and might reflect
increased use of communal swimming venues (e.g., lakes, rivers,
swimming pools, and
water parks) by young children.
REF: MMWR, Surveillance
Summaries, Volume
54, No. SS-1, January
28, 2005

EPA
Launches New Spanish Website
The EPA has launched a new consolidated Spanish
website as part of its ongoing effort to provide information in this
language. Lead poisoning, asthma triggers, and pesticide management are
among the topics at the site (www.epa.gov/espanol). (EPA OPP Update, 1/21/05).
REF: Chemically Speaking, February 2005.


EPA Provides List
of Alternatives to CCA-Treated Wood for Residential Use
In response to requests from consumers regarding
available alternatives to chromated copper arsenate- (CCA) treated wood
for use in residential settings, EPA has made available online
information about arsenic-free wood preservatives and alternative
building materials. Effective December 31, 2003, wood can no longer be
treated with CCA for most residential uses. Since the December 31
cancellation, EPA has received many queries about what alternatives to
CCA-treated wood are available. Making this information available via
the Web empowers consumers to make educated building material choices.
You can find more information on these alternatives at
http://www.epa.gov/oppad001/reregistration/cca/alternativestocca.htm.
This list of alternatives to CCA is being
introduced as part of our effort to reorganize and update the CCA Web
pages on epa.gov. By reorganizing the information on those pages from a
chronological to topical structure, EPA hopes to make it easier for the
general public and other stakeholders to find the information they
seek. The new and improved CCA page is available online at http://www.epa.gov/pesticides/antimicrobials/reregistration/cca/.
REF: EPA
News Release.


The National
Toxicology Program Announces the Release of the 11th
Report on Carcinogens
The Department of Health and Human Services
released its Eleventh Edition of the Report on Carcinogens today,
adding seventeen substances to the growing list of cancer-causing
agents, bringing the total to 246. For the first time ever, viruses are
listed in the report: hepatitis B virus, hepatitis C virus, and some
human papillomaviruses that cause common sexually transmitted diseases.
Other new listings include lead and lead compounds, X-rays, compounds
found in grilled meats, and a host of substances used in textile dyes,
paints and inks.
The full report is available at the NTP website http://ntp.niehs.nih.gov.
REF: National Toxicology Program Press Release, Jan 31, 2005.

CHEERS
Everyone agrees
that the protection of children’s health is a primary goal of pesticide
regulation. The problem has been difficulty in determining and
measuring the risks. To address this problem, EPA initiated the Children’s Health Environmental Exposure
Risk Study (CHEERS). It is not a study of the top party
schools. The two-year study will collect information about
families who volunteered to participate in the project. The data will
include household pesticide use and potential exposure for children up
to three years old. The participating families will be asked not to
change their normal routine of pesticide use (unless spraying their
kids is the status quo). The scientists will collect samples of food
from the homes and urine samples from the children. The information
will be used to estimate the children’s exposure to pesticides and
phthalates. Phthalates are chemicals commonly used to make plastics;
there is some concern that phthalates can interfere with human
hormones.
At the request of EPA, the Batelle Memorial
Institute, the University of North Carolina, the Duval County (Florida)
Health Department, and the University of Florida reviewed the
scientific merit of the study and the protection of human subjects. All
of the institutions approved the study in 2004. The EPA has decided to
send the project design out for another external, independent review by
an expert panel made up of members of the Science Advisory Board, the
Science Advisory Panel, and the Children's Health Protection Advisory
Committee.
The results of this study could drive significant
pesticide decisions. The risks to children may be greater or less than
we currently think. It is imperative that the design of this study be
above criticism by groups that are either pro- or anti- pesticide.
You will find more information about CHEERS at
this address. http://www.epa.gov/cheers/basic.htm
You cannot volunteer to participate unless you live in the Florida
study area.
REF: Georgia Pest Management Newsletter, December 2004/Volume
27, No. 12

Surgeon General's
Advisory on Alcohol Use in Pregnancy
In February 2005, the U.S. Surgeon General issued
an Advisory on Alcohol Use in Pregnancy to raise public awareness about
this important health concern. Research demonstrates that prenatal
alcohol exposure can result in a spectrum of birth defects that can
affect a child's growth, appearance, cognitive development, and
behavior.
Fetal alcohol spectrum disorders are preventable if a woman abstains
from drinking alcohol while pregnant.
In 2003, approximately 10% of pregnant women
reported alcohol use, with 4% of them reporting binge drinking. In
addition, nearly 55% of women who might become pregnant report drinking
alcohol, and more than 12% report binge drinking. Because
approximately 50% of pregnancies are unplanned, prevention efforts
should target not only pregnant women and women planning a pregnancy
but also women of childbearing age who are sexually active and not
using an effective form of birth control. This new advisory reaches out
to this broader group of women and urges them to abstain from alcohol.
The Surgeon General's Advisory on Alcohol Use in
Pregnancy is available at http://www.hhs.gov/surgeongeneral/pressreleases/sg02222005.html.
Additional information about alcohol use and pregnancy is available
from CDC at http://www.cdc.gov/ncbddd/fas,
the National Institute on Alcohol Abuse and Alcoholism at http://www.niaaa.nih.gov, and the
Substance Abuse and Mental Health Services Administration at http://www.fascenter.samhsa.gov.
REF: MMWR, March 11, 2005, 54(09).
Michigan Hunter
Contracts Bovine TB
A hunter who cut his hand while gutting a deer is
the first living person diagnosed with a cutaneous form of bovine
tuberculosis found in some northern Michigan deer and cattle, officials
said on Jan 6, 2005. The man killed the deer in Alcona County in
October 2004 and sought medical attention after spotting telltale
lesions in the animal's chest cavity, said TJ Bucholz, spokesman for
the Michigan Department of Community Health. He said the man is being
treated and is expected to recover.
The community health department laboratory in
Lansing identified the TB strain early in January. The strain also was
found during an autopsy of an elderly person who died in 2002, but it
was not the cause of death, Bucholz said. Different strains of the
disease have been found in 8 people from foreign countries in Michigan
since 1995. The disease -- rare in humans but highly contagious in
animals -- has saddled Michigan farmers with costly testing
requirements and hampered their ability to market cattle in neighboring
states during the decade-long outbreak in the northern Lower Peninsula.
The Centers for Disease Control and Prevention have
no statistics on bovine TB in humans, spokeswoman Karlie Stanton said.
But studies show that eradication programs and milk pasteurization have
reduced the number of cases over the years. Human cases usually are
caused by breathing infected barn air or drinking unpasteurized milk
from a sick cow. It's extremely rare to get the disease the way it
happened in the latest case [cutaneous transmission].
"This appearance of bovine TB in a human underscores
the human health risk of the disease in free-ranging deer," said Janet
Olszewski, state community health director. "People should not consume
wild animals that appear or are confirmed to be sick, regardless of the
circumstance."
The case also shows the importance for hunters of
wearing gloves while gutting deer and washing hands afterwards, said
Rebecca Humphries, director of the Michigan Department of Natural
Resources. The infected man was not wearing gloves.
The USDA revoked Michigan's status as free of bovine
TB in 2000. State officials have ordered testing of the state's nearly
one million cattle, and some herds have undergone multiple testing,
said Bridget Patrick, coordinator of the state's eradication task
force. Cattle on 34 farms and 483 deer have tested positive. Goats and
elk are among other animals susceptible to the deadly sickness, which
attacks the lungs and sometimes the digestive tract. Michigan has asked
the USDA to restore the TB-free status in the Upper Peninsula, where no
cases have been found. The federal agency in 2004 upgraded most of
Michigan to "modified accredited advanced," the rating immediately
below TB-free. 11 counties and parts of 2 others in the northern Lower
Peninsula retained a lower rating.
REF: From AnimalNet-L January 12, 2005


Lab
Notes from CAHFS
(California Animal Health and Food
Safety Laboratory System)
Cattle
Nitrate toxicosis
was the cause of death of 90 dry cows and heifers on a dairy. The three
affected pens had 40-100% death rate after consuming a diet solely of
alfalfa hay received the day before. Ocular fluid nitrates were high,
and the hay was heavily contaminated with
lambsquarter, a nitrate accumulator.
Hay nitrate was 5%.
English Yew (taxus baccata) was the
cause of sudden death in two, 3-year-old Limousin heifers that had been
fed
cemetery clippings. The
plant was identified in the rumen and the toxic principle taxine found.
In cattle, the estimated toxic dose is 0.36-0.7 g fresh plant/kg body
weight, or 0.75 lbs to kill an adult cow.
Horses
Oleander poisoning
was diagnosed in a 19-year-old Quarter horse. Oleander bushes were
present around the property. The stomach and colon content were
positive for the cardiac glycoside, oleandrin.
All parts of the oleander, dried and fresh,
are considered toxic. Four other cases were diagnosed in horses
throughout California over a period of six months.
English Yew (taxus
baccata) was also found at 2.25% in stomach contents of a
2-year-old Thoroughbred gelding that died with an hour of eating.
Fresh, dried, or stored leaves contain the toxic and can be lethal.
Taxine is reported to affect the heart conduction resulting in a
bradycardia, eventually leading to heart stoppage and death. In most
cases, animals die within minutes, without much premonitory signs.
Death may occur a few minutes after eating the plant or up to several
days later.
Llamas
Oleander toxicosis
caused the death of three adult llamas after exposure to oleander
trimmings. Oleandrin was identified in the stomach contents.
REF: Lab Notes, 17(1), Fall 2004.

!! CLICK ON THE PIG !!



Cow Study Yields Surprises about Source, Amount of 'Dairy Air' Pollution
Potentially Harmful Fluoride Levels Found in Some Instant Teas
DPR Releases 2003 Pesticide Use Data; Director Emphasizes Reduced-risk Strategy
DPR Reports on Environmental Justice Project, Releases Illness Statistics for 2003
FDA Assesses New Report on Acrylamide
USDA Pesticide Data Program to Release 2003 Data
Lead Poisoning Associated with Use of Litargirio
Salmonellosis Associated with Pet Turtles
































